
With decades of experience in medical device contract manufacturing and medical OEM/ODM services, our team has built strong capabilities in on-time delivery, medical device design capability, and product customization. Over the years, we observed subtle yet significant changes in the demands for medical partitions within hospitals and healthcare facilities. The urgent needs during the COVID-19 pandemic accelerated this transformation, inspiring us to independently develop a lightweight, extendable medical partition — our first step toward building an in-house brand beyond OEM projects.
Market Insights and User Pain Points Driving Innovation
Medical partitions, though seemingly simple, have been dominated by two design directions:
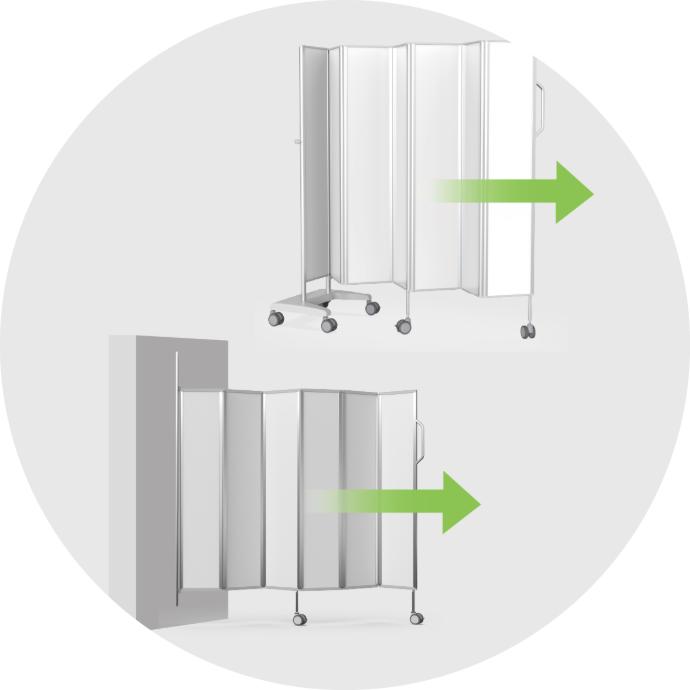
Extendable partitions
Highly flexible, adaptable to different spatial requirements, but often complex and costly.

Lightweight partitions
Easy for single-person deployment and quick mobility, suitable for dynamic clinical environments, but usually lack extendable features.

Prevents droplet transmission
The screen must be easy to clean and disinfect, while effectively blocking droplet flow.
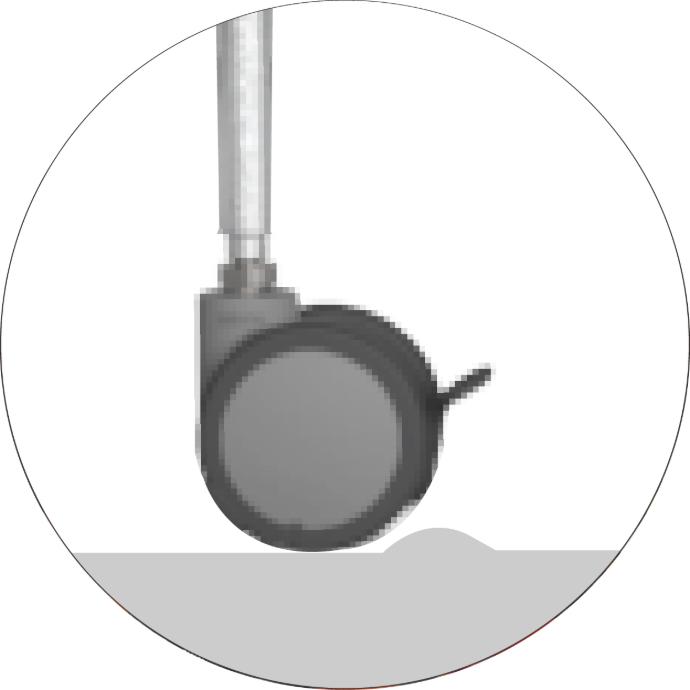
Lightweight and easy to move
Able to absorb uneven floor surfaces during movement, preventing wheel damage.
To bridge this gap, our R&D team proposed a new approach: maintaining structural lightweight design while integrating extendability. Through material innovation, we replaced heavy full-metal sheet structures with composite panels that offer both higher rigidity and improved processability. The connecting mechanism was redesigned to reduce parts, improving both assembly efficiency and structural stability.
We also introduced multi-stage extendable structures with smooth-rolling mobility systems, ensuring that healthcare workers can deploy and store the partition single-handedly. This design successfully passed patent approval, marking a milestone as our first medical product with independent intellectual property rights.
Pandemic-Era Adaptation and Rapid Deployment
During the COVID-19 crisis, hospitals urgently required rapid spatial reconfiguration for isolation and screening. We optimized our design into an anti-epidemic partition, adding antimicrobial coatings and replaceable transparent window modules. This version was widely deployed in emergency triage zones, testing stations, and isolation wards — ultimately making it to the central government’s procurement list.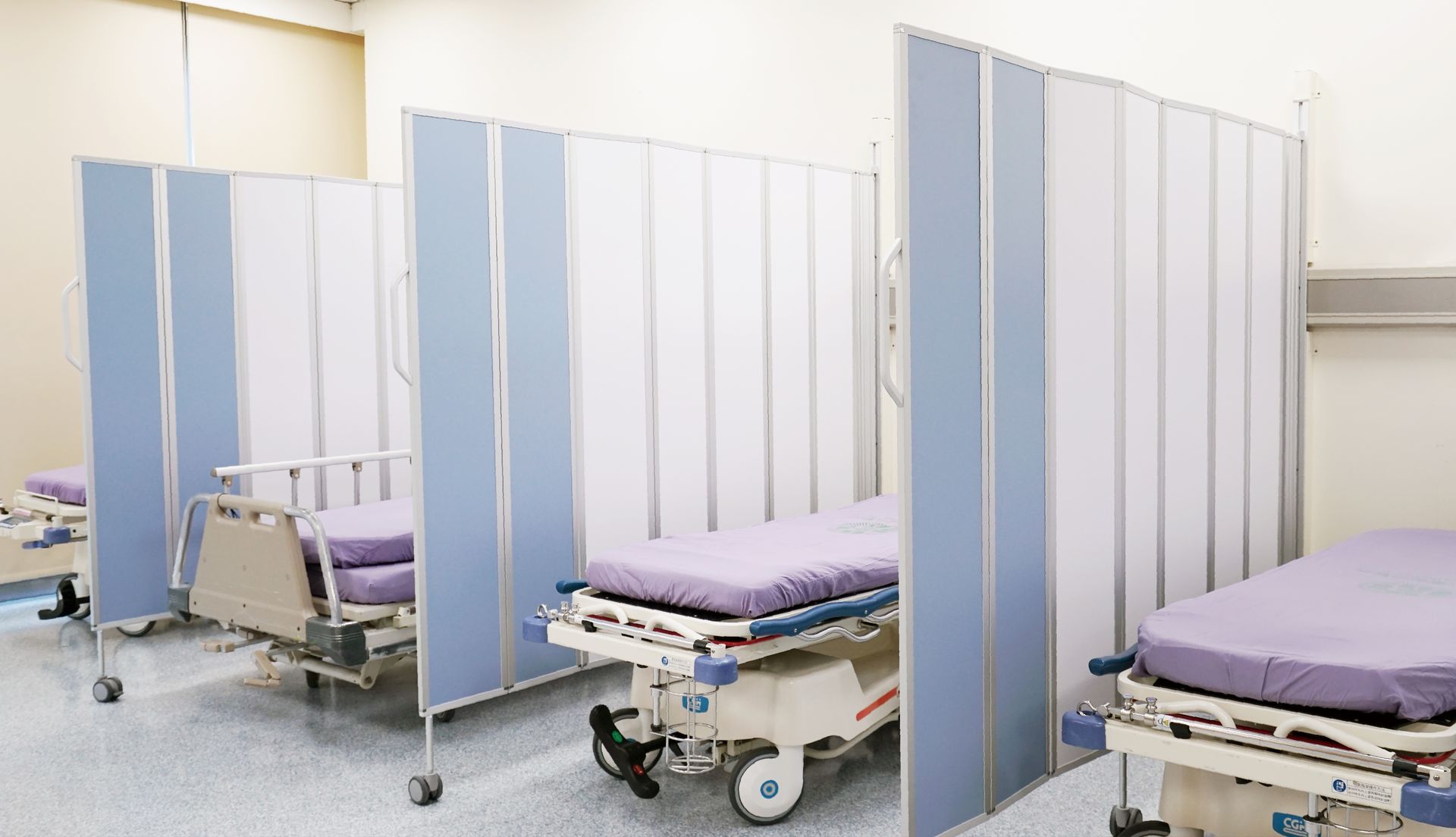
Expanding into International Markets
Following its success in Taiwan, the product gained attention from global medical OEM partners and healthcare distributors, especially in Japan and the UK, where mobility and spatial flexibility are valued. After passing product trials, the partition entered OEM cooperation and certification processes, with plans for further global expansion.
Conclusion
This project represents not just a product launch, but a strategic transformation — moving from pure medical OEM manufacturing toward independent innovation. By combining design capabilities, manufacturing expertise, and market-oriented thinking, we deliver solutions that meet the evolving needs of the global medical industry.